Introduction:
Batteries have become an integral part of our daily lives, powering an extensive array of devices that range from smartphones to electric vehicles. Despite their ubiquity, the inner workings of batteries are often shrouded in mystery for many. In this exhaustive guide, we will embark on a journey to demystify the intricate mechanisms behind how batteries work, exploring the fundamental principles, types of batteries, and their applications in various domains.
Understanding the Basics:
1. Electrochemical Reactions:
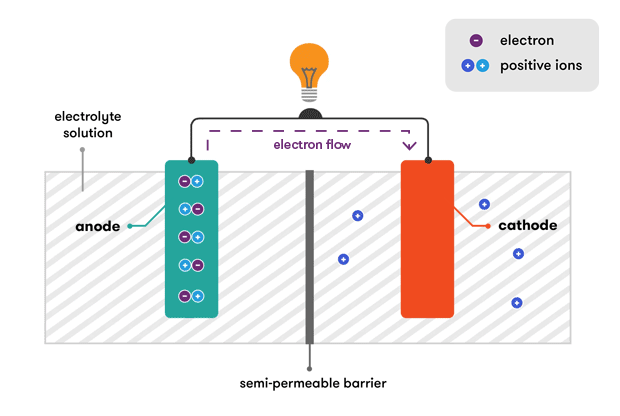
At the heart of every battery is an electrochemical reaction that converts stored chemical energy into electrical energy. This process involves the movement of electrons between two materials – anode and cathode – through an electrolyte.
2. Anode and Cathode:
The anode and cathode are the two electrodes within a battery. The anode is the electrode where oxidation (loss of electrons) occurs, while the cathode is where reduction (gain of electrons) takes place. These reactions are essential for the flow of electrons, creating an electric current.
3. Electrolyte:
The electrolyte serves as a medium for the transport of ions between the anode and cathode. It can be a liquid, gel, or solid, and its composition plays a critical role in determining the battery’s performance and safety.
Types of Batteries:
1. Alkaline Batteries:
- Common in household devices.
- Zinc and manganese dioxide react electrochemically.
- Relatively inexpensive and widely available.
2. Lithium-ion Batteries:
- Prevalent in smartphones, laptops, and electric vehicles.
- Lithium cobalt oxide or other lithium-based compounds in the cathode.
- High energy density and rechargeable.
3. Lead-Acid Batteries:
- Found in vehicles, uninterruptible power supplies (UPS), and renewable energy systems.
- Lead dioxide and sponge lead are used in the cathode and anode, respectively.
- Robust, inexpensive, but relatively heavy.
4. Nickel-Cadmium (NiCd) Batteries:
- Historically common but being phased out due to environmental concerns.
- Nickel oxide hydroxide cathode and cadmium anode.
- Known for a “memory effect.”
5. Nickel-Metal Hydride (NiMH) Batteries:
- Used in hybrid vehicles, power tools, and consumer electronics.
- Nickel oxide hydroxide cathode and metal hydride anode.
- Higher energy density than NiCd, less prone to memory effect.
6. Zinc-Carbon Batteries:
- Basic and inexpensive; often used in low-drain devices.
- Zinc anode, manganese dioxide cathode, and ammonium chloride or zinc chloride electrolyte.
The Battery Life Cycle:
1. Charge and Discharge:
- Batteries undergo cycles of charge and discharge during normal operation.
- Charging involves the movement of ions from cathode to anode, storing energy.
- Discharging releases stored energy, moving ions from anode to cathode.
2. Capacity and Voltage:
- The capacity of a battery refers to the amount of energy it can store, measured in ampere-hours (Ah).
- Voltage is the electric potential difference between the anode and cathode, determining the force with which electrons flow.
3. Self-Discharge:
- Batteries experience self-discharge over time, losing charge even when not in use.
- Factors like temperature, battery chemistry, and storage conditions influence self-discharge rates.
4. Depth of Discharge (DoD):
- DoD measures the percentage of a battery’s capacity that has been utilized during a discharge cycle.
- Managing DoD can impact the overall lifespan of a battery.
Environmental Considerations:
1. Recycling Challenges:
- Certain battery chemistries pose recycling challenges due to toxic materials.
- Lead-acid batteries have a high recycling rate, while proper disposal of lithium-ion batteries is critical.
2. Advancements in Sustainable Batteries:
- Researchers are exploring eco-friendly alternatives and sustainable battery technologies.
- Solid-state batteries and improvements in recycling processes contribute to a greener future.
Applications of Batteries:
1. Consumer Electronics:
- Powering smartphones, laptops, cameras, and wearable devices.
2. Transportation:
- Electric vehicles rely on advanced battery technologies for clean and efficient mobility.
3. Renewable Energy Storage:
- Batteries play a crucial role in storing energy generated from renewable sources like solar and wind.
4. Medical Devices:
- Powering various medical devices, from hearing aids to implantable pacemakers.
5. Military and Aerospace:
- Batteries are essential in military applications, aerospace technology, and space exploration.
Emerging Trends and Future Prospects:
1. Solid-State Batteries:
- Solid-state batteries promise higher energy density, improved safety, and longer lifespans.
2. Wireless Charging:
- Advancements in wireless charging technologies aim to enhance convenience and efficiency.
3. Energy Storage for the Grid:
- Large-scale batteries contribute to energy grid stability, storing excess energy for peak demand periods.
4. Biodegradable Batteries:
- Researchers explore biodegradable materials to create environmentally friendly battery options.
Conclusion:
The world of batteries is both complex and fascinating, encompassing diverse chemistries, applications, and innovations. Understanding how batteries work not only sheds light on the devices we use daily but also underscores the importance of sustainable practices in the development and disposal of these essential power sources. As technology continues to advance, batteries will play a pivotal role in shaping a greener and more energy-efficient future.