Introduction:
Aestivation, a term less commonly known than its counterpart hibernation, is a fascinating biological phenomenon that revolves around the concept of dormancy. Unlike hibernation, which involves a state of reduced activity during the cold winter months, aestivation is a strategic adaptation employed by certain organisms to endure prolonged periods of heat and aridity. In this comprehensive exploration, we embark on a journey into the world of aestivation, unraveling its mechanisms, the diverse array of species that utilize this strategy, and the ecological significance of this enigmatic dormancy state.
I. Definition and Characteristics of Aestivation:
A. Definition:
- Aestivation, derived from the Latin word “aestas” meaning summer, refers to a state of suspended animation or dormancy that organisms enter to survive harsh environmental conditions, particularly extreme heat and aridity.
- Adaptation to Adverse Conditions: Aestivation is an adaptive response that allows organisms to conserve energy, reduce metabolic activity, and endure challenging environmental conditions.
B. Key Characteristics:
- Metabolic Depression: Aestivating organisms experience a significant reduction in metabolic rate, conserving energy resources during the dormant period.
- Behavioral Changes: Aestivation is often accompanied by altered behavior, including reduced activity levels and a state of torpor or deep sleep.
II. Aestivation in Different Taxa:
A. Invertebrates:
- Snails and Slugs: Certain terrestrial gastropods aestivate to avoid desiccation during dry and hot periods.
- Insects: Some insects, like the African desert ant (Cataglyphis fortis), aestivate to cope with high temperatures and scarcity of food and water.
B. Amphibians:
- Frogs and Toads: Aestivation is observed in certain amphibians, especially in arid regions, where they burrow into the ground to escape extreme temperatures.
- Lungfish: Some species of lungfish aestivate in dried-up mud during droughts, entering a state of metabolic dormancy.
C. Reptiles:
- Turtles and Tortoises: Aestivation is a common strategy for certain turtle and tortoise species, enabling them to survive in arid environments by burrowing or seeking shelter.
- Lizards: Some lizard species aestivate to endure high temperatures and water scarcity, reducing their metabolic activity during the dormant phase.
D. Mammals:
- Rodents: Certain desert-dwelling rodents, like the kangaroo rat, aestivate to conserve water and energy during hot and dry periods.
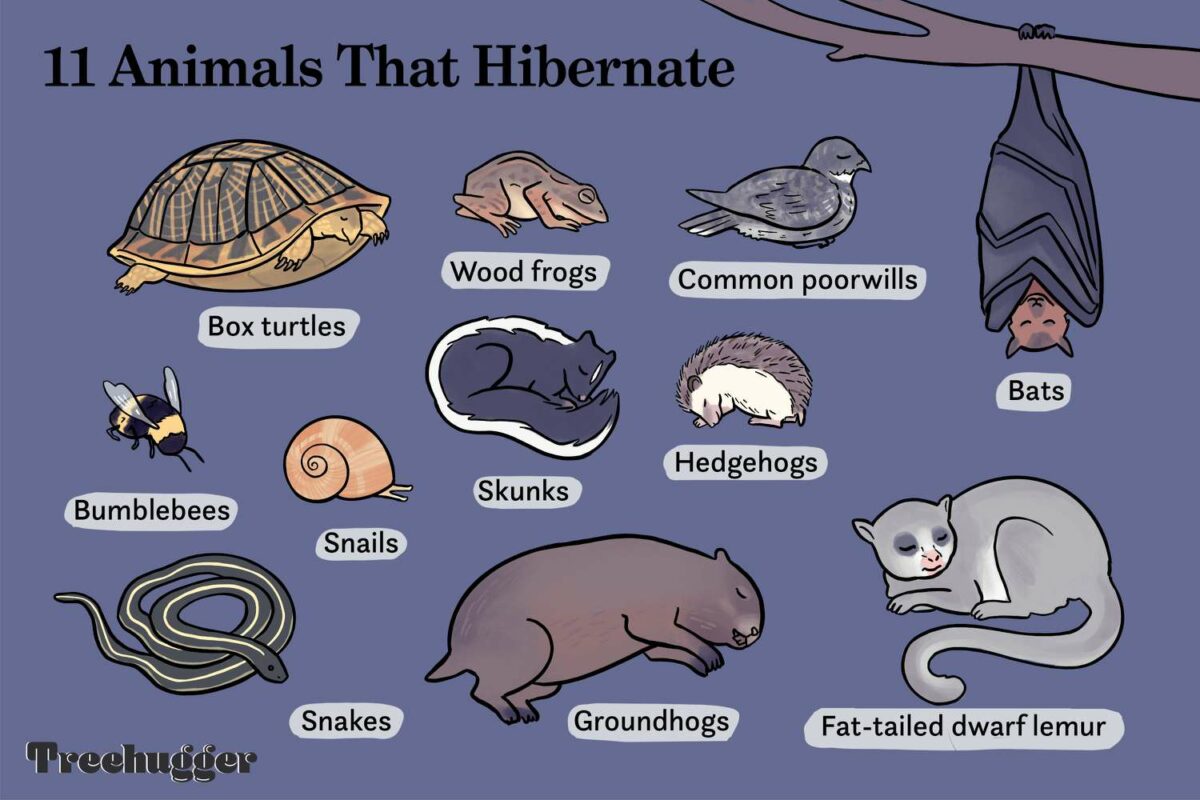
- Lemurs: Aestivation has been observed in the fat-tailed dwarf lemur (Cheirogaleus medius), allowing it to survive seasonal changes in Madagascar.
III. Physiological and Behavioral Adaptations:
A. Metabolic Adjustments:
- Metabolic Rate Reduction: Aestivating organisms undergo a substantial decrease in metabolic rate, conserving energy and minimizing water loss.
- Dehydration Tolerance: Some aestivators exhibit enhanced tolerance to dehydration, enabling them to endure extended periods without water.
B. Burrowing and Shelter Seeking:
- Substrate Selection: Aestivating organisms often choose specific substrates, such as soil or burrows, to create a microenvironment that buffers against extreme external conditions.
- Torpor and Inactivity: Behavioral adaptations include a state of torpor or inactivity, reducing movement and conserving energy during aestivation.
IV. Environmental Triggers and Cues:
A. Temperature:
- High Temperatures: Aestivation is commonly triggered by elevated temperatures, providing a means for organisms to escape the detrimental effects of heat stress.
- Seasonal Variation: Aestivation may be synchronized with seasonal changes, aligning with periods of extreme heat and reduced water availability.
B. Photoperiod:
- Day Length: Changes in day length or photoperiod can serve as cues for initiating aestivation, particularly in species that respond to seasonal variations in light.
- Hormonal Regulation: Hormones play a crucial role in coordinating the physiological changes associated with aestivation in response to environmental cues.
V. Ecological Significance:
A. Survival Strategies:
- Adaptation to Harsh Environments: Aestivation allows organisms to survive in environments with challenging conditions, enhancing their ecological resilience.
- Resource Conservation: By reducing metabolic activity and conserving energy, aestivating species can endure periods of resource scarcity, contributing to overall ecosystem stability.
B. Biotic Interactions:
- Predator Avoidance: Aestivation can serve as a strategy to avoid predation, as aestivating organisms may be less vulnerable to predators during their dormant state.
- Competition Mitigation: Aestivation can help mitigate competition for resources during periods of environmental stress, allowing aestivating species to avoid direct competition with other active organisms.
VI. Challenges and Threats:
A. Habitat Loss and Fragmentation:
- Altered Environments: Human-induced alterations to natural habitats, including urbanization and deforestation, can disrupt aestivation sites and jeopardize the survival of aestivating species.
- Climate Change:
a. Altered Temperature Patterns: Changes in temperature patterns due to climate change may impact the timing and duration of aestivation, affecting the synchronization of physiological responses.
b. Water Scarcity: Aestivation may become more challenging for species that rely on water conservation strategies as climate-induced water scarcity intensifies.
VII. Conservation Implications:
A. Habitat Preservation:
- Protected Areas: Preserving natural habitats and creating protected areas that encompass aestivation sites are crucial for the conservation of species utilizing this adaptation.
- Landscape Connectivity: Maintaining landscape connectivity ensures the availability of suitable aestivation habitats and promotes genetic diversity.
B. Climate Change Mitigation:
- Sustainable Practices: Implementing sustainable land-use practices and mitigating climate change can help preserve the environmental conditions necessary for aestivating organisms.
- Research and Monitoring: Continuous research and monitoring of aestivating species provide insights into their adaptations and help guide conservation efforts in the face of changing environmental conditions.
VIII. Future Research Directions:
A. Molecular and Genetic Studies:
- Genomic Exploration: Investigating the genetic basis of aestivation can provide insights into the molecular mechanisms governing physiological adaptations.
- Comparative Genomics: Comparative genomics across species employing aestivation can reveal common genetic elements and adaptive pathways.
B. Climate Change Resilience:
- Physiological Responses: Research on how aestivating species respond to changing climatic conditions can inform strategies for conservation and climate change adaptation.
- Ecosystem-Level Effects: Understanding the broader ecological implications of altered aestivation patterns can guide conservation strategies at the ecosystem level.
IX. Conclusion:
Aestivation, a captivating strategy for survival in the face of extreme heat and aridity, showcases the remarkable adaptability of diverse organisms across various taxa. From invertebrates to mammals, these creatures enter a state of suspended animation, navigating harsh environmental conditions through metabolic adjustments and behavioral adaptations. As we unravel the intricacies of aestivation, we gain not only a deeper understanding of the physiological mechanisms at play but also an appreciation for the role these dormant periods play in maintaining ecological balance. However, the challenges posed by habitat loss, climate change, and human-induced alterations underscore the need for concerted conservation efforts to preserve the habitats and conditions that support aestivating species. In the continuum of scientific inquiry, the study of aestivation stands as a testament to the resilience and ingenuity of life in adapting to the ever-changing dynamics of the natural world.