Introduction: Determining the concentration of a solution is a fundamental task in chemistry and various scientific disciplines, essential for preparing solutions with precise compositions, conducting experiments, and analyzing reaction kinetics. Solution concentration refers to the amount of solute dissolved in a given quantity of solvent, typically expressed as moles per liter (M), mass per volume (g/L), or percentage (%). In this comprehensive guide, we delve into the intricacies of solution concentration determination, exploring the principles, methods, and considerations involved in accurately quantifying solution concentrations for a wide range of applications.
- Understanding Solution Concentration: Solution concentration is a measure of the relative amount of solute dissolved in a solvent, expressed as the ratio of solute quantity to solvent volume or mass. Common units of concentration include molarity (M), molality (m), normality (N), mass/volume (g/L or mg/mL), and percentage (%). The choice of concentration unit depends on the nature of the solute, solvent, and specific application, with each unit offering advantages and limitations in terms of precision, accuracy, and ease of use.
- Methods of Solution Preparation: Preparing solutions with accurate and precise concentrations requires careful measurement and calculation to achieve the desired solute-to-solvent ratio. Various methods can be used to prepare solutions, including volumetric dilution, mass-based dilution, serial dilution, and standard solution preparation. Volumetric glassware, such as volumetric flasks, pipettes, and burettes, are commonly used for accurate measurement of liquid volumes, while analytical balances are employed for precise weighing of solid solutes.
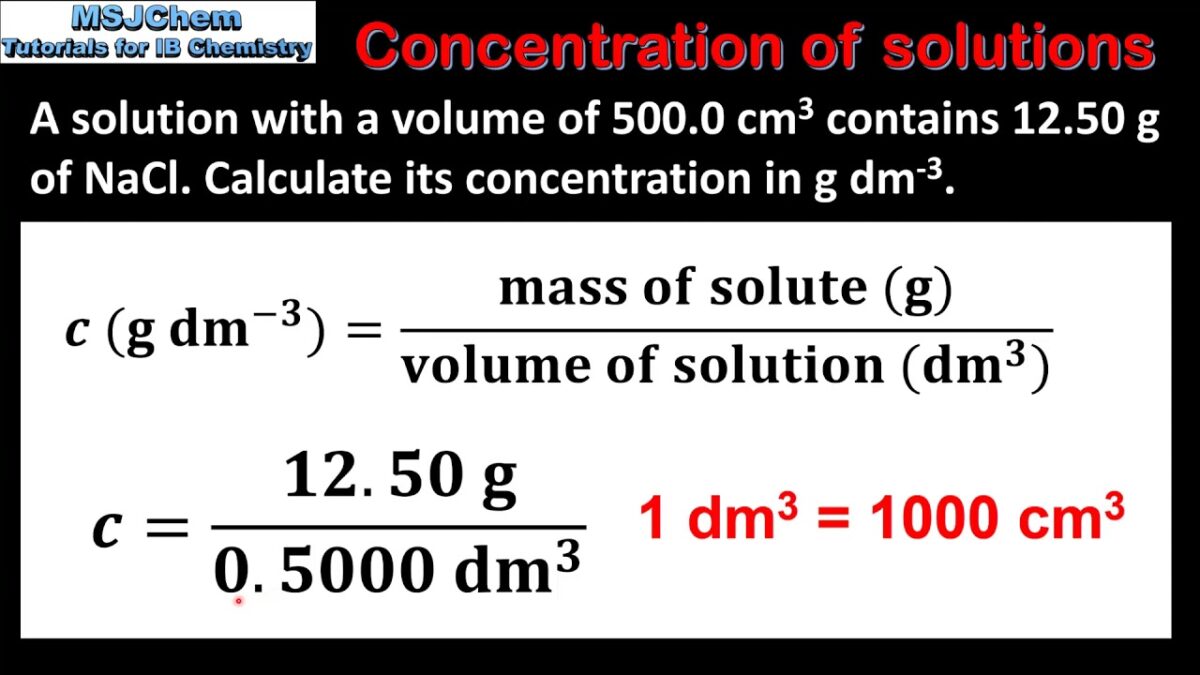
- Calculation of Solution Concentration: Once a solution is prepared, its concentration can be calculated using appropriate formulas and equations based on the chosen concentration unit. For example, molarity (M) is calculated by dividing the number of moles of solute by the volume of solution in liters (M = mol/L), while mass/volume concentration (g/L or mg/mL) is calculated by dividing the mass of solute by the volume of solution in liters or milliliters (g/L or mg/mL = g/mL or mg/mL). For dilute solutions, the dilution factor must be taken into account when calculating final concentrations after dilution.
- Spectrophotometric Analysis: Spectrophotometric analysis is a powerful technique for determining solution concentrations based on the measurement of light absorption or transmission properties of a solution. In UV-Visible spectrophotometry, the absorbance of a solution at a specific wavelength is directly proportional to its concentration, allowing for quantitative analysis of solute concentrations in solution. Standard calibration curves or Beer-Lambert law equations can be used to relate absorbance values to solute concentrations over a defined range.
- Titration Methods: Titration is a classical analytical technique used to determine solution concentrations by reacting a solution of known concentration (titrant) with a solution of unknown concentration (analyte) until the reaction reaches equivalence. Common types of titration methods include acid-base titrations, redox titrations, and complexometric titrations, each of which relies on specific chemical reactions and indicators to determine endpoint or equivalence point. Titration curves and stoichiometric calculations are used to calculate analyte concentrations based on the volume and concentration of titrant added.
- Chromatographic Techniques: Chromatographic techniques, such as high-performance liquid chromatography (HPLC) and gas chromatography (GC), are widely used for quantitative analysis of solution concentrations in complex mixtures. These techniques separate individual components of a solution based on their interactions with a stationary phase and mobile phase, allowing for precise quantification of solute concentrations. Detector signals or peak areas are correlated with solute concentrations using calibration standards or external calibration methods.
- Electrochemical Methods: Electrochemical methods, such as potentiometry, coulometry, and voltammetry, are employed for quantitative analysis of solution concentrations based on electrical properties of redox reactions or ion concentrations. These techniques involve measuring electrical potentials, currents, or charges generated during electrochemical reactions between analyte species and electrodes. Faraday’s laws of electrolysis and Nernst equation are used to calculate analyte concentrations from measured electrochemical parameters.
- Quality Assurance and Validation: Ensuring the accuracy and reliability of solution concentration measurements requires robust quality assurance practices and validation procedures. Calibration of instruments, verification of measurement techniques, and traceability to certified reference materials are essential for maintaining measurement accuracy and consistency. Quality control checks, proficiency testing, and inter-laboratory comparisons help identify and mitigate potential sources of error or variability in concentration determination.
- Data Analysis and Reporting: Analysis of solution concentration data involves statistical analysis, uncertainty estimation, and data interpretation to derive meaningful conclusions and make informed decisions. Statistical methods, such as regression analysis, confidence intervals, and hypothesis testing, are used to assess the reliability and significance of concentration measurements. Results are reported with appropriate units, significant figures, and uncertainty values to convey the precision and accuracy of the concentration determination process.
- Applications and Practical Considerations: Solution concentration determination finds applications in various fields, including analytical chemistry, pharmaceuticals, environmental monitoring, food and beverage analysis, and biotechnology. Accurate quantification of solution concentrations is essential for ensuring product quality, compliance with regulatory requirements, and safety of consumer products. Practical considerations, such as sample preparation, matrix effects, interference, and detection limits, must be taken into account when selecting appropriate methods and techniques for concentration determination.
Conclusion: Mastering the art of solution concentration determination is a multidimensional endeavor that requires a combination of theoretical knowledge, practical skills, and analytical techniques. By understanding the principles, methods, and considerations involved in quantifying solution concentrations, scientists, chemists, and researchers can achieve accurate and reliable results for a wide range of applications. Through continuous education, training, and innovation, solution concentration determination remains a cornerstone of analytical chemistry and scientific inquiry, driving advancements in research, technology, and industry.